History and Timeline of Puritan Medical Products
Our History
A Century of Improving Life in Ways Large and Small
The world and the industries we serve have changed in ways people from 1919 could never have imagined. Here’s a look at some of the bigger moments down through the years.

Open for Business
Lloyd Cartwright establishes the Minto Toothpick Company in Saginaw, MI where he produces and sells mint-flavored toothpicks. 1920 brought a change of plan when toothpicks became difficult to find in Saginaw. The production operation was moved to Guilford, Maine where the needed white birch was plentiful. This move allowed for an expanded product line and soon thereafter a name change as well, to Hardwood Products Company.
1920 – Ice Cream on a Stick
The Good Humor ice cream company says that confectioner Harry Burt invents ice cream on a stick, and Good Humor is granted a patent in 1923.
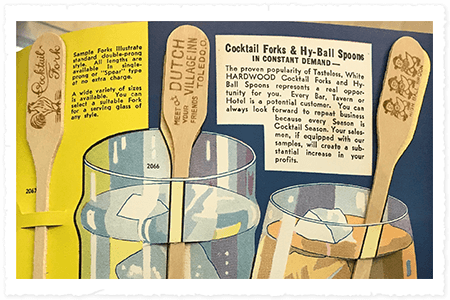
Product Diversification
Puritan starts developing a range of products, such as ice cream sticks and spoons, candy sticks, steak markers, cocktail forks, tongue blades and manicure sticks. Products were sold under the brand names GoldBond and Trophy, which are the same brands used today by our sister company Hardwood Product Co., LLC.
1923 - Cotton Swab Invented
After watching his wife affix wadded cotton to the tip of a toothpick, Leo Gerstenzang creates a solution to cleaning hard-to-reach spots (like the ear).

On to Medical Woodenware
Puritan enters the medical woodenware market with the introduction of tongue depressors and aseptic wood applicators.
1938 – Flu Vaccine Invented
Jonas Salk and Thomas Francis develop the first flu vaccine. The vaccine, along with the use of cotton swabs for specimen collection, helps protect US forces against the flu during World War II.

Puritan Brand Trademarked
Hardwood Products Company trademarks the well-known Puritan® brand. All Puritan medical products bear the name and logo.

New Address
The Hardwood Products Co. maintained its sales and administration office in Michigan until 1950. It was then decided to consolidate the company’s administrative, sales and manufacturing divisions and locate all operations to Guilford, ME.

Disaster and Recovery
After fire in 1958 destroys the plant and warehouse, dedicated owners and employees immediately start building a new facility that opens in 1960. Most of the specialized machinery was built by company personnel on site as it was not available for purchase on the open market.

Move Toward Specialization
Puritan decides to become more heavily involved in the production of medical and healthcare products. The decision is made to enter the cotton tipping and sterile pack market laying the groundwork for a future in the development of swabs and specimen collection devices.

FDA Approval Secured
Puritan registers as a medical device manufacturer with the federal Food and Drug Administration.

Adapting to Market Demands
Puritan brings fiber carding equipment online to more efficiently prepare cotton, rayon and polyester fibers to better address the needs of the healthcare industry.
1984 – Reaching for the Stars
President Ronald Reagan calls on NASA to build an international space station within 10 years. Puritan supplies the project with samples for various uses.

A Tip of the Hat
The pilgrim hat is added to the Puritan logo to reflect and embody the dedication, honor and determination of the Puritan pilgrims.

On to Industrial Market
Puritan rolls out new technology, producing foam-tipped applicators for industrial uses, as well as for medical and specimen collection applications.

International Certification Achieved
The global International Standard Organization (ISO) certifies Puritan’s production facilities. CE marking, signifying approval under European Union standards, follows in 1998.
2000 – Forensics in our Homes
CSI premieres on CBS, launching the use of forensic science – and cotton swabs! – into the popular TV police drama genre and America’s cultural consciousness.

Second Product Patented
Puritan releases its second patented product, the DM™ Wound Measuring device. It joins the Popule®, a sterile foam swab for use in the field for collection of trace DNA.

From One Manufacturer to Two
Hardwood Products Company remains as Hardwood Products Company LP but restructures to manufacture product as two separate but affiliated companies in Guilford. Puritan Medical Products Company LLC is born. Production remains in Guilford and under founding family ownership.
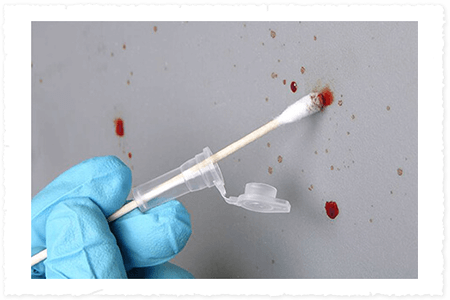
Evidence of Ingenuity
Puritan introduces Cap-Shure® DNA specimen collection device to the world of forensics.
Line Expansion
Puritan’s PurSwab line expands, adding a new array of specialty swabs for lint-sensitive applications.
Innovation Continues
DNA collection devices are now offered “free” of human DNA contamination.
2009 – WHO Calls, Puritan Answers
The World Health Organization applies its highest alert level to the worldwide swine flu pandemic, Puritan goes all-out to boost production of specimen collection devices. That same year, home ancestry DNA kits enter the marketplace.

Game-changing Products
Puritan debuts its patented line of high-performance flocked swabs. PurFlock Ultra ® and HydraFlock ® swabs offer superior collection and rapid release properties. EnviroMax ®, EnviroMax Plus ®, and ESK ® Sampling Kit products address the environmental sampling needs of the food and drug industries.

Innovation in Transport Mediums
UniTranz-RT® and Opti-Swab® — Puritan’s superior media for viral and bacterial transport, coupled with Puritan’s well-established specimen collection swabs, are launched.
Award-winning Technology
Puritan unveils its transport media system while also receiving an Innovation & Technology Award from the Institute for Maine Family-Owned Businesses.
2018 – Worst Flu Season in Nearly a Decade
Puritan supplies diagnostic products to combat the worst flu season in nearly a decade.

Celebrating 100 Years
Unveiling a 100-year anniversary logo and a yearlong celebration campaign, while charging ahead with continuously improving manufacturing capabilities and growing our workforce.
COVID-19 Response Team
Puritan faced the challenge head-on when called upon by the White House to increase capacity to meet the needs of the US COVID-19 testing program. With the support of the Task Force, bringing both financial and technological assets, we have expanded production into two new manufacturing facilities focused on producing foam and our patented flocked swabs.
Present & Future
Puritan will always be in the forefront, meeting the needs of emerging technologies. Our continuing R&D and facility expansion allows our continued leadership in single-use products for patient care, diagnostics, controlled environments, environmental sampling and forensic and genetics markets.
Have a Question?
Contact us to speak with one of our knowledgeable and friendly product specialists.
Contact Us

